As devoted dog owners, we’ve all wished our four-legged companions could stay with us longer. Understanding the science behind how dogs age not only helps us appreciate the time we have with them but also provides insights into how we can optimize their health throughout their lives. Recent advances in veterinary science and genetics have revealed fascinating details about the biological mechanisms that govern canine aging and why different breeds age at remarkably different rates.
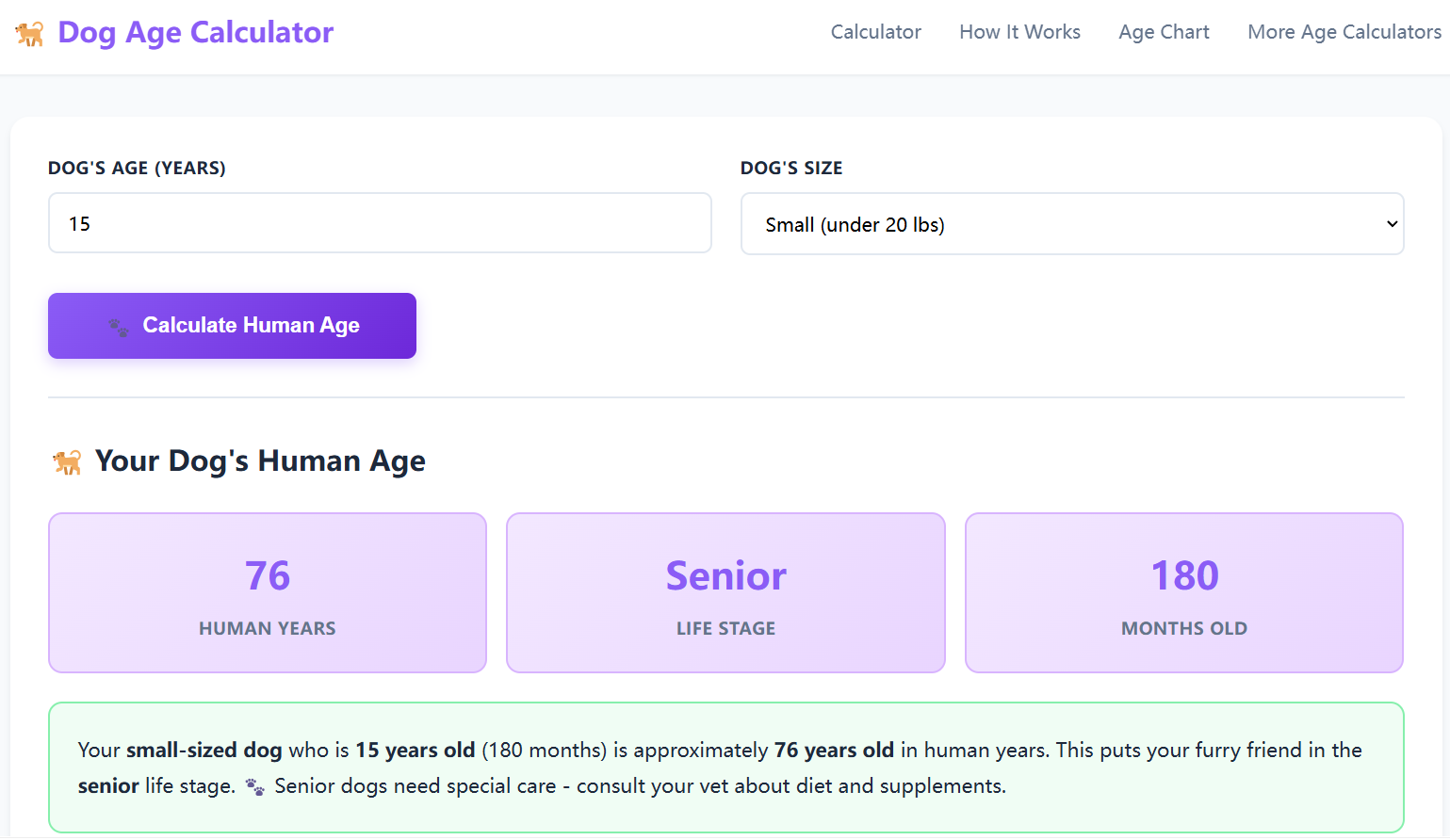
Can you calculate how old your dog is?
The Biology of Canine Aging
At its core, aging in dogs follows many of the same biological pathways observed in other mammals, including humans. However, dogs experience this process in a compressed timeframe, making them valuable subjects for aging research.
Cellular Aging and Oxidative Stress
Like all living organisms, dogs age at the cellular level through a process involving oxidative stress and cellular damage. As cells metabolize oxygen to produce energy, they generate reactive oxygen species (free radicals) as byproducts. Over time, these molecules damage cellular components including DNA, proteins, and lipids. While dogs possess natural antioxidant defenses, these systems become less efficient with age, allowing cumulative damage to accumulate.
This oxidative damage affects multiple organ systems, contributing to age-related conditions such as cognitive decline, joint deterioration, and cardiovascular disease. The rate at which this damage accumulates varies significantly between individuals and breeds, influenced by both genetic and environmental factors.
Telomeres and Cellular Senescence
One of the most significant discoveries in aging research involves telomeres—protective caps on the ends of chromosomes. Each time a cell divides, these telomeres shorten slightly. Eventually, when telomeres become critically short, cells can no longer divide and enter a state called senescence, essentially retiring from active function.
Research has shown that dogs with shorter telomeres relative to their age tend to have shorter lifespans. Interestingly, larger dog breeds tend to have shorter telomeres than smaller breeds, which may partially explain their reduced longevity. This relationship between telomere length and lifespan has made telomere research a promising avenue for understanding and potentially extending canine longevity.
Hormonal Changes
The endocrine system undergoes significant changes as dogs age. Growth hormone and insulin-like growth factor 1 (IGF-1) levels, which are crucial during development, continue to influence aging throughout life. Studies have revealed that dogs with lower IGF-1 levels tend to live longer, a finding that has important implications for understanding breed differences in lifespan.
Why Size Matters: The Breed Longevity Paradox
Perhaps the most intriguing aspect of canine aging is the inverse relationship between body size and lifespan—a phenomenon that contradicts patterns observed in most other mammals. In the wild, larger animals typically live longer than smaller ones. Elephants outlive mice, and whales can live for centuries. Yet in dogs, the opposite holds true: smaller breeds consistently outlive their larger counterparts.
The Numbers Tell the Story
On average, small dog breeds live 12-16 years, while giant breeds often live only 7-10 years. A Chihuahua may enjoy 15 years or more, while a Great Dane rarely reaches 10. This dramatic difference occurs despite sharing 99.8% of their DNA, making it a unique model for studying the genetics of aging.
Accelerated Growth, Accelerated Aging
The key to understanding this paradox lies in the developmental differences between breeds. Large breed puppies grow at an extraordinarily rapid rate, sometimes increasing their body weight by 100-fold in their first year. This accelerated growth comes at a biological cost.
Research has demonstrated that larger dogs age faster at the cellular level. A study analyzing thousands of dogs found that for every 4.4 pounds of body weight, a dog’s life expectancy decreases by approximately one month. The mechanisms behind this include:
Higher metabolic rates during growth: Large breed puppies require more energy and resources to fuel their rapid development, potentially leading to earlier depletion of cellular resources.
Increased oxidative stress: The intense metabolic activity during growth produces more free radicals, causing earlier cellular damage.
Earlier onset of age-related diseases: Large breeds develop conditions like cancer and joint diseases at younger ages than small breeds, suggesting their biological aging clock runs faster.
The IGF-1 Connection
Insulin-like growth factor 1 (IGF-1) plays a central role in this size-lifespan relationship. This hormone regulates growth and development, with larger breeds producing significantly more IGF-1 than smaller breeds. However, elevated IGF-1 throughout life has been linked to:
- Increased cancer risk
- Faster cellular aging
- Earlier onset of age-related decline
Smaller breeds with naturally lower IGF-1 levels appear protected from these accelerated aging processes, contributing to their extended lifespans.
Genetic Factors in Canine Aging
The remarkable diversity in dog breeds provides a unique opportunity to study the genetic basis of aging. All modern dog breeds descended from wolves within the past 15,000-40,000 years, yet selective breeding has created enormous variation in size, appearance, and lifespan.
Longevity Genes
Researchers have identified several genetic variants associated with canine longevity:
IGF-1 gene variants: Mutations in genes controlling IGF-1 production directly correlate with body size and lifespan. Small breeds carry genetic variants that limit IGF-1 production, while large breeds have versions that promote higher levels.
DNA repair genes: Variations in genes responsible for repairing cellular damage influence how well dogs can maintain their genetic integrity over time. Some breeds appear to have more efficient DNA repair mechanisms.
Mitochondrial genes: Since mitochondria are the cellular powerhouses that generate energy and produce free radicals, genetic variations affecting mitochondrial function can significantly impact aging rates.
The Dog Aging Project
One of the most ambitious studies of canine aging is the Dog Aging Project, launched in 2018. This longitudinal study is following tens of thousands of dogs throughout their lives, collecting data on genetics, environment, lifestyle, and health outcomes. The project aims to:
- Identify genetic markers associated with healthy aging
- Understand environmental factors that promote longevity
- Develop interventions to extend healthspan (years of healthy life)
- Provide insights applicable to human aging research
Early findings from this project have already revealed connections between physical activity, diet quality, and longevity, while confirming the strong genetic component to lifespan differences between breeds.
Breed-Specific Aging Patterns
Different breeds don’t just live for different lengths of time—they also experience unique patterns of aging and age-related diseases.
Small and Toy Breeds
Breeds like Chihuahuas, Toy Poodles, and Yorkshire Terriers often remain youthful well into their teen years. However, they face specific age-related challenges:
- Dental disease (due to crowded teeth)
- Tracheal collapse
- Heart valve degeneration
- Patellar luxation
Medium Breeds
Medium-sized dogs like Beagles, Cocker Spaniels, and Border Collies typically enjoy a balanced aging process, often remaining active and healthy until their final years. Their moderate growth rate appears to offer a sweet spot between the extremes of very small and very large breeds.
Large and Giant Breeds
Great Danes, Mastiffs, and Saint Bernards face the most compressed lifespan, with aging signs appearing earlier:
- Earlier onset of cancer (the leading cause of death in large breeds)
- Joint diseases and arthritis from supporting heavy bodies
- Heart disease, particularly dilated cardiomyopathy
- Earlier cognitive decline
Current Research and Future Directions
The field of canine aging research is experiencing rapid advancement, driven by both veterinary interest and the relevance to human aging studies.
Rapamycin and Longevity
One of the most promising areas of research involves rapamycin, an immunosuppressant drug that has extended lifespan in laboratory animals. The Dog Aging Project is conducting clinical trials to determine whether low doses of rapamycin can safely extend both lifespan and healthspan in dogs. Early results suggest improvements in cardiac function in middle-aged dogs, though long-term effects are still being studied.
Senolytic Therapies
Scientists are investigating drugs called senolytics that can selectively eliminate senescent cells—the “retired” cells that accumulate with age and contribute to inflammation and tissue dysfunction. Removing these cells in laboratory animals has reversed some aging effects, and similar approaches are being explored for dogs.
Regenerative Medicine
Stem cell therapies and regenerative medicine approaches are being developed to repair age-related damage to joints, organs, and tissues. These treatments show promise for managing arthritis, cognitive decline, and other age-related conditions in dogs.
Epigenetic Aging Clocks
Researchers have developed “epigenetic clocks” that can determine a dog’s biological age (as opposed to chronological age) by analyzing chemical modifications to DNA. These tools can predict remaining lifespan and identify dogs aging faster or slower than expected, potentially enabling earlier interventions.
Optimizing Your Dog’s Healthspan
While genetics play a major role in determining how long a dog will live, environmental factors and care significantly influence healthspan—the number of years spent in good health.
Nutrition
Maintaining a healthy weight is one of the most impactful interventions. Studies show that dogs kept at optimal body condition live significantly longer than overweight counterparts. Moderate caloric restriction has been shown to extend lifespan by reducing oxidative stress and inflammation.
Exercise and Mental Stimulation
Regular physical activity and cognitive engagement help maintain muscle mass, joint flexibility, and brain health. Dogs with active lifestyles show slower cognitive decline and better mobility in their senior years.
Preventive Healthcare
Regular veterinary check-ups, dental care, and early detection of age-related diseases enable interventions that can extend both lifespan and quality of life. Blood work, monitoring for breed-specific conditions, and staying current on vaccinations all contribute to healthy aging.
Environmental Enrichment
A stimulating environment with social interaction, novel experiences, and problem-solving opportunities supports cognitive health and emotional well-being throughout a dog’s life.
The science of dog aging reveals a complex interplay between genetics, biology, and environment. While we cannot change the genetic hand our dogs have been dealt—whether they’re tiny Chihuahuas or gentle giants—understanding the mechanisms of aging empowers us to make informed decisions about their care.
As research continues to unlock the secrets of canine longevity, we move closer to interventions that could extend not just the length of our dogs’ lives, but more importantly, the quality of their years. Until then, the best approach remains providing excellent nutrition, regular exercise, preventive healthcare, and above all, the love and companionship that makes the time we share with our dogs so precious.
The bond between humans and dogs is one of the oldest and most enduring interspecies relationships. By understanding the science behind how our canine companions age, we honor that bond and work toward ensuring they can remain by our sides, healthy and happy, for as long as possible.